Application of PVC Injection Molds in Medical Device Manufacturing
Medical-grade polyvinyl chloride (PVC) has become a core raw material for disposable medical devices due to its good biocompatibility, chemical corrosion resistance, and cost advantages. It is widely used in infusion catheters, blood storage devices, dialysis accessories, and other products. Its compatibility with injection molds needs to meet dual requirements: at the material level, non-toxic additives such as calcium-zinc composite stabilizers should be used to ensure that plasticizer migration meets GB 9685 standards; at the mold level, it must match PVC’s characteristics of poor thermal stability (narrow processing temperature window) and high melt viscosity to avoid harmful decomposition products during molding.
Data shows that medical-grade PVC injection molded products account for over 35% of the disposable medical device market, with 80% relying on customized injection molds, placing strict requirements on mold material selection and structural design.
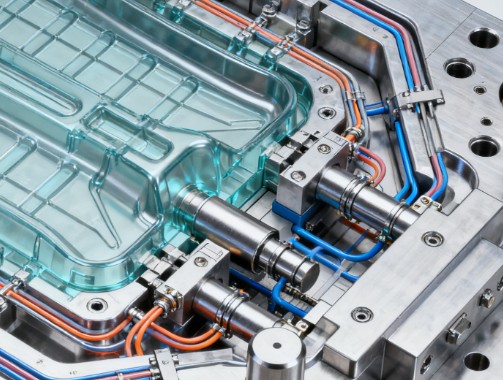
1. Core Design Specifications for Medical PVC Injection Molds
1.1 Mold Materials and Heat Treatment Standards
The mold base should use corrosion-resistant steel. After quenching and tempering, template hardness should be controlled at HRC 32-36; cores and cavities, after vacuum quenching, must reach HRC 58-62 to resist erosion from PVC melt. For transparent catheters, fixed mold cores require precision polishing with a surface roughness Ra value ≥ 0.20μm; liquid polishing may be used to improve finish and prevent microbial adhesion.
1.2 Key Structural Design Points
Gating System Optimization: Transparent products prioritize hot runner systems to reduce weld lines. Cold runner lengths for flexible PVC products should be shortened by over 30%, with overflow wells at feed ports to eliminate air entrapment.
Ventilation and Cooling: Long tubular catheter molds need 0.015mm-wide axial vents along cores. Cooling systems use multiple independent water channels, with temperature differences between moving and fixed molds controlled within ±2°C to prevent internal stress from uneven cooling.
Automation Compatibility: For mass production, molds must integrate automatic ejection and gate cutting mechanisms, with ejection system clearance ≤ 0.01mm for seamless robotic integration.

2. Precise Control of Injection Molding Parameters
2.1 Temperature and Pressure Ranges
Based on PVC characteristics, barrel temperatures are sectionally controlled at 170-200°C, nozzle temperatures at 180-210°C, and mold temperatures (for rigid PVC) at 30-60°C. Injection pressure is set at 80-150MPa, with holding pressure at 50-100MPa to ensure filling while preventing shrinkage. Critical note: Screw barrels must be cleaned before shutdown to avoid equipment corrosion from residual material decomposition.
2.2 Solutions for Common Defects
| Defect Type | Cause | Solution |
|---|---|---|
| Surface yellowing/smoking | Excessive temperature or long residence time | Reduce material temperature by 5-10°C; shorten cycle time |
| Bubbles/pinholes | High moisture in raw materials | Dry at 80°C for 2 hours; deepen vents |
| Dimension instability | Uneven cooling | Optimize water channels; stabilize injection pressure |
| Brittle fracture | Excessive internal stress | Anneal at 60-70°C for 1-2 hours |

3. Typical Medical Device Applications
3.1 Infusion Catheter Molds
Using pinpoint gate feeding with 360° circumferential cooling cores ensures catheter wall thickness tolerance ≤ ±0.02mm. Mold parting surfaces use stepped sealing with clearance ≤ 0.005mm to eliminate flash, meeting sterile requirements.
3.2 Hemodialysis Accessory Molds
Cavity surfaces undergo passivation for anticoagulation. Internally heated hot runner nozzles increase weld line strength by 40%, ensuring leak resistance at 0.3MPa pressure.
4. Compliance and Quality Control
Medical PVC injection mold production must follow GMP standards, with fully enclosed workshops, constant temperature-humidity systems, and harmful gas recovery. Raw materials are transported via vacuum-sealed systems to prevent contamination; mold maintenance prohibits rust removers, with regular UV disinfection.
During quality checks, molds require calibration every 500,000 cycles. Cores with wear exceeding 0.01mm must be repaired to ensure compliance with ISO 13485.
